The use of stainless steel on explosion-proof equipment
In industrial plants such as petrochemical, chemical and, in general, in places with potentially explosive atmosphere and with possible...
In industrial plants such as petrochemical, chemical and, in general, in places with potentially explosive atmosphere and with possible presence of gas or explosive and aggressive dust, different materials may be used, depending on installation, explosiveness and corrosion characteristics.
The materials used today in these areas for the realization of electrical equipment, lighting fixtures, fittings and components, are mainly divided into two categories:
- Ferrous: aluminium with a low content of copper, cast iron, brass, carbon steel, stainless steel
- Non Ferrous: mass polyester reinforced with glass fibers and graphite, polycarbonate, borosilicate glass
In this article, we talk about the use of stainless steel as a raw material for the production of electrical equipment and materials, suitable for installation in potentially explosive environment and its ability to withstand corrosion caused by weather and aggressive substances.
1. Some types of stainless steels
- Martensitic stainless steels. These steels have carbon content between 0.10 and 0.50% (with peaks of more than 1%) and chromium content from 11 up to 18%. They are the only steels that can take hardening and, therefore, increase the mechanical characteristics (tensile strength, yield strength, hardness) by heat treatment. Good aptitude to plastic deformation, especially when heated.
- Ferritic stainless steels. These steels, with ferritic stable structure at any temperature, have indicatively a chromium content between 16% and 28% and a very low carbon content, usually less than 0.10%, which can reach up to 0,35% only when the chromium is at the upper limit. They are easy to work by plastic deformation, both hot and cold, can be machined with machine tools (in particular resulphurized types). They can be easily welded, in particular in case of resistance welding (spot welding and rolling).
- Austenitic stainless steels. These steels, with stable austenitic structure at any temperature, are those that, in addition to the chromium (from 16% up to 26%), contain nickel with a content comprised between 6% and 22% and a very low carbon content (less than 0,10%). Even these steels cannot be hardened but it’s possible to increase their tensile properties with work hardening by cold deformation (rolling, pressing, etc.). Great workability by cold deformation and with machine tools. They can be welded both by resistance and electric arc welding. Depending on their chemical composition and characteristics of use, they can be divided into two groups:
- Austenitic Cr-Ni, (AISI 304 and 304L), characterized by the presence of 16-20% Cr and 7-12% Ni, with the possible addition of other elements such as sulphur and selenium which facilitate machining by chip removal, or titanium or niobium as stabilizers of carbon to prevent the formation of chromium carbides. They haven’t high mechanical properties at ambient temperature, but they keep significant at very low temperatures. They have also a good resistance to stress and corrosion in almost all aggressive environments. For this reason they are very used in the food and chemical industries, in medical appliances, kitchen tools and cookware.
- Austenitic Cr-Ni-Mo, (AISI 316 and 316L), have a chemical composition of Chrome from 16 up to 18%, of Nickel from 10 up to 18% and of Molybdenum 6.2%. The presence of Molybdenum gives to these steels a particular resistance to pitting corrosion, thus allowing the use even in chemically aggressive environment and also in the presence of solutions containing chlorine ions. The excellent corrosion resistance of this category of steels permits their use in the manufacture of installations for the processing of nitrates, of cellulose, of natural and synthetic fibers. They are also used in shipbuilding and in the food industry with machining of particularly aggressive products (fruit pickles, fruit juices) and in the wine industry, for the storage of white wines and vermouth particularly sensitive to any trace of iron.
- Austenitic Cr-Ni, (AISI 304 and 304L), characterized by the presence of 16-20% Cr and 7-12% Ni, with the possible addition of other elements such as sulphur and selenium which facilitate machining by chip removal, or titanium or niobium as stabilizers of carbon to prevent the formation of chromium carbides. They haven’t high mechanical properties at ambient temperature, but they keep significant at very low temperatures. They have also a good resistance to stress and corrosion in almost all aggressive environments. For this reason they are very used in the food and chemical industries, in medical appliances, kitchen tools and cookware.
- Austenitic Cr-Ni-Mo, (AISI 316 and 316L), have a chemical composition of Chrome from 16 up to 18%, of Nickel from 10 up to 18% and of Molybdenum 6.2%. The presence of Molybdenum gives to these steels a particular resistance to pitting corrosion, thus allowing the use even in chemically aggressive environment and also in the presence of solutions containing chlorine ions. The excellent corrosion resistance of this category of steels permits their use in the manufacture of installations for the processing of nitrates, of cellulose, of natural and synthetic fibers. They are also used in shipbuilding and in the food industry with machining of particularly aggressive products (fruit pickles, fruit juices) and in the wine industry, for the storage of white wines and vermouth particularly sensitive to any trace of iron.
2. Standard austenitic stainless steels
Stainless steels most commonly used are typically defined as "standard austenitic". They are the 1.4301 (known as AISI 304), which contains from 17 up to 19.5% of chromium and from 8 up to 10.5% of nickel and a content of carbon ≤ 0.07%. The 1.4401 (known as AISI 316), which contains from 16.5 up to 18.5% of chromium and from 8 up to 10.5% of nickel and a carbon content of ≤ 0.07%. These types of steels were manufactured, in the past, with a higher carbon content considerably with implications on the corrosion behaviour. In fact, the carbon present in steel reacts with the chromium causing the precipitation of chromium carbides at the grain boundaries, in thermal cycles such as in the heat-affected zones by welding. This local loss of chromium from the boundary region of the carbide particles allows a preferential inter crystalline corrosion attack, defining these types of steel "sensitized or affection from weld decay".
The types with low carbon content of these classes are the 1.4306 (AISI 304L) and 1.4404 (AISI 316L). They have a lower carbon content, ≤ 0.03%, to avoid problems relating to the corrosion resistance in the presence of welds, and they are preferred compared to steels described above.
The reference Standard is the EN 10088, which consists of three parts:
- EN 10088-1: Part 1 - List of stainless steel.
- EN 10088-2: Part 2 - Stainless steel - Technical delivery conditions for sheet/plate and strip of corrosion resisting steels for general purpose.
- EN 10088-3: Part 3 - Stainless steel – Technical delivery conditions for semi-finished products, bars, rod and sections for general purposes.
The designation system of EN 10088 is based on the European steel number and the name of the steel.
For example, the AISI 316L type has the number of steel 1.4404, in which:
1 denotes the material (stainless steel)
44 denotes the group of stainless steel
04 denotes the quality
The name of the steel 1.4404 (AISI 316L) is X2CrNiMo17-12-2 in which its chemical composition, is:
X denotes high alloy steel
2 denotes a low carbon content (≤ 0,03%)
CrNiMo denotes the symbols of the main elements in the alloy (Cr=Chrome, Ni=nickel; Mo=molybdenum)
17-12-2 denotes the percentage value of the main elements in the alloy, in the identical sequence of the description of symbols.
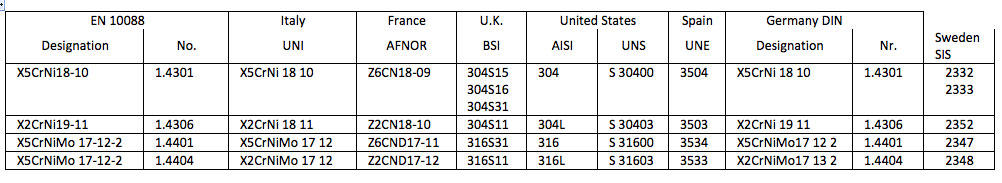
Table 1 - Designation of stainless steels adopted in different countries, compared to the European Community Standard EN 10088.
The Table 2 shows the chemical composition of austenitic stainless steels which are usually employed by our company and are normally quoted in the construction specifications for use in hazardous areas, according to the classification of the EN 10088-1 standard.
Table 2 - (values extracted from the rule EN 10088-1:2005 – table 4 - Chemical composition, cast analysis of austenitic corrosion resisting steels)

In which: C= Carbon; Si= Silicium; Mn= Manganese; P= Phosphorus; S= Sulfur; N= Nitrogen; Cr= Chrome; Cu= Copper; Mo= Molybdenum; Nb= Niobium; Ni= Nickel; Other= unspecified
3. Austenitic steels used for the construction of electrical equipment
Austenitic steels, which are usually used for the construction of electrical equipment, suitable for environments with risk of explosion and in the presence of aggressive substances, are mainly:
- AISI 304 It’s a founder of austenitic stainless steels. It has good corrosion resistance and good mechanical characteristics. Used in the pharmaceutical and food industries.
- AISI 304L It differs from AISI 304 for the low carbon content (C≤0,03%), which considerably increases the resistance to corrosion. Because of the low carbon content of the mechanical characteristics, they are slightly inferior to AISI 304 ones.
- AISI 316 Compared to AISI 304 contains molybdenum with percentage of about 2,5% and a higher percentage of nickel. These elements give to the steel superior mechanical characteristics and a higher resistance to corrosion at high temperatures. Used in the processes of machining and of installations in contact with seawater.
- AISI 316L It differs from AISI 316 for the low carbon content (C ≤ 0,03%). It has a better corrosion resistance maintaining good mechanical characteristics. Used for the construction of electrical equipment and for the construction of distribution panels in environments with explosion risk and the presence of gases or dusts, corrosive agents and high temperatures.
4. Passivation
The presence, sometimes combined, of acids, whose aggressive action is exacerbated where the combustion air is contaminated by the presence of other corrosive substances, can cause severe injuries of the surface. Specifically, in harsh environments such as chemical, petrochemical, oil and most industrial and tertiary environments, the acids used, if dissolved in the air, are considered to be highly corrosive. The use of austenitic stainless steel AISI 316L, ensures resistance to attack of corrosive components.
Stainless steels are defined in this way by the fact that, in the presence of an oxidizing environment (therefore also in contact with the air that contains oxygen), a protective layer is formed on their surface consisting of adsorbed oxygen. This is the so-called phenomenon of passivation.
This phenomenon in stainless steel, in ideal conditions, occurs naturally and immediately. As soon as this metal is scratched or cut, removing one part, the protective layer regenerates immediately.
This protection can also be artificially and optimally induced carrying out a particular chemical treatment by immersing the products in sequence, first in pickling acid and, subsequently, in a passivating acid. This treatment enhances very significantly the corrosion resistance, making this invisible layer, which has the thickness of a few atoms (about 0.002 microns) and which reform spontaneously, an excellent barrier to oxidation and corrosion. Essential condition for the formation of a protective layer is the presence of a sufficient amount of chromium.
Why they are called stainless steels if they oxidize?
This term does not correspond to the true nature of these metals. In fact they oxidize. This means that they have the possibility, thanks to the content of alloying elements, in particular the percentage of Chromium (Cr), of "self passivate": create a layer of invisible oxides of molecular size which protect the underlying metal from corrosion by external agents.
This layer guarantees the coverage of the metal, although locally occurred abrasion or removal of the protective film. So it’s necessary to let the material to exchange with the environment a quantity of oxygen, so that it can be considered in the best conditions of passivation.
This concept is very important for a good resistance in time and to counteract adequately the different cases of corrosion.
Of course, this passive film can be more or less resistant and more or less anchored to the material depending on the concentration of chromium in the alloy and also the possible presence of other elements such as nickel (Ni), molybdenum (Mo), titanium (Ti), etc.
Fig. 1 Diagram of film formation process
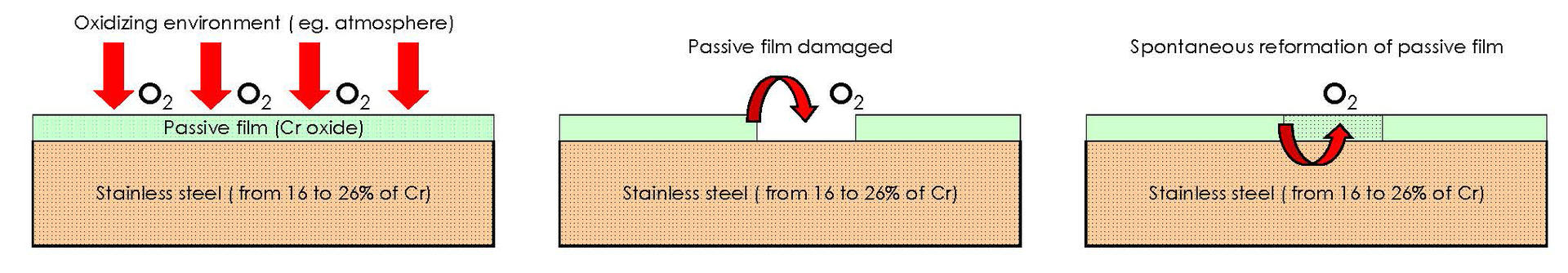
Fig. 2 Detail on the formation of passive film damage (Source Lamiera, April 2008)

5. The surface finishes
The appearance of the surface finish is critical to avoid the use of too noble alloys. It appears intuitively that the more a surface is smooth, the more the possibility of anchoring of an aggressive element decreases. The passivity film that covers it gives the corrosion resistance of stainless steels. In fact, this film will be formed much more easily and will be much more stable better is the substrate finish.
The resistance to corrosion, in principle, will be the higher, the greater will be the smoothing of the surface, that is the lower will be the surface roughness of stainless steel. In addition, may be considered other factors such as the bond that exists between the cleanability and the greater or lesser smoothness of a surface.
The main types of finishes are:
- 2B finish: the finish through a cold laminate film (skin pass) with polished cylinders. It’s bright and silvery grey and it’s the most common finish for cold-rolled sheet.
- BA finish: it’s a finish of sheets and cold-rolled strip obtained by the thermal annealing treatment, recrystallization or solubilisation in an inert atmosphere after rolling and the subsequent degreasing. Given the type of heat treatment, the material is not oxidized and, therefore, there is not the pickling operation, thus maintaining the very bright and shiny aspect, almost perfectly speculate that derives from the cold rolling, possibly followed by further laminating skin pass.
6. Corrosion, the enemy of stainless steels
One of the characteristics of stainless steels and one of the reasons for the continuing spread of their use is certainly their resistance to corrosion. However, it would be wrong to think that such materials may hold wherever and however to corrosion.
Their resistance depends on many factors such as:
- Aggressive nature of the agent (type, concentration, pH...)
- Temperature of aggressive agent
- Metal surface finishing
- Installation conditions
We can say that the chlorides (CI) are the main enemies of stainless steel, because that can break the film of passivation and prevent its reformation. The concentration of chloride ions and the acidity (pH) are, together with the temperature, the determining factors when choosing the type of stainless steel.
The phenomenon of corrosion, if looked closer, may be very different and, therefore, it’s important to understand the mechanism and the causes that generate it, in order to take appropriate action in the choice of materials to use.
The most dangerous types of corrosion are usually those located, which may give rise to the formation of cracks, holes, and fissures.
On the contrary, generalized phenomena are the less dangerous because progressive and fairly constant in time. This allows determining, with sufficient approximation, the duration of the material subject to corrosion.
However, determine quite precisely the duration of an equipment placed in an aggressive environment it’s a very difficult task because the parameters that determine it are extremely complex and diversified.
The main types of corrosion are:
- The fretting corrosion occurs when two non-lubricated surfaces, in the atmospheric environment, are in contact: fretted each other and subjected to vibration or, anyway, to a cyclic continuous rubbing. The phenomenon is formed due to localized mechanical destruction of the passive layer occurring with the appearance of small pitting shaped ulcers on the surface. To overcome this type of corrosion it’s necessary to modify, as far as is possible, the movement between the parts acting to prevent rubbing or, at least, to increase the amplitude of it. A good lubrication with fluids of appropriate viscosity, extended to the whole contact surface, or interposing between the parts with good plastic characteristics may be another solution.
- The pitting corrosion is the most corrosive phenomenon known on stainless steels and it’s a localized corrosion that occurs on the surface with small holes, in certain cases invisible to the human eye, surrounded by a halo of dark colour and by a series of deep underlying cavity. A triggering precedes the corrosive phenomenon, which tears the protective oxide film due to the surface state and the environment in which it operates. It’s a type of corrosion that, for its characteristics, is very dangerous, since it acts in depth on very narrow areas. These effects can easily escape to a visual inspection; this is why the damage progresses smoothly as to perforate the part attacked. Factors that may facilitate the appearance of corrosion are: surface roughness (as a smooth surface is, much less is subject to corrosion), surface chips or ferrous contamination. Typical environments suitable to develop pitting corrosion are the seawater and, in general, the water containing chlorine ions, especially if stagnant. In general, the maximum corrosion resistance will be using special steels with high molybdenum content. Other regimens that can put in place are: ensure the highest decontamination of surfaces from ferrous traces, employ corrosion inhibitors, avoid the presence of interstices between the surfaces of the equipment in contact with the aggressive agent.
- The erosion corrosion is originated by the flow of a fluid, even mildly corrosive, when on the stainless steel surface are present solid particles capable of causing mechanical wear. This is the case of brackish or exhaust water containing abrasive particles in suspension. The attack occurs much more severely than greater is the amount of solid in suspension in the fluid. It therefore occurs, in particular, in correspondence of tight bends of pipes, in the "T" grafts of pipes, in the pump rotors and the turbine blades. To overcome this type of corrosion is necessary a careful design of the parts so as to avoid turbulent motions of the fluid, sudden changes in direction of flow velocity, avoiding or reducing the presence of suspended solids.
- The Galvanic corrosion occurs when, in the presence of an electrolyte (an acid solution, saline, atmospheric humidity), two different metallic elements are directly connected to each other with electrical continuity, forming a real "stack". The more anodic will corrode faster. Therefore, it is discouraged join with aluminium or common steel nails or screws, stainless steel parts immersed in corrosive environments, as will be discouraged also contaminate the stainless steels with more anode materials (for example carbon steel) given that small traces of these corrode more quickly. It will always be appropriate instead, when there are the conditions for the occurrence of galvanic corrosion, join stainless steels with other stainless steel parts.
- The crevice corrosion is a type of localized corrosion and can arise when equipment has interstices between two coupled surfaces. It is manifested in the presence of solutions containing ions reducing agents, such as the chlorine ion, within the interstices created by the surface contact between several artefacts, including organic. The fluid inside the interstice is not countered with the outer one. Initially, the solution inside the interstice is equal to the outside and the passive anode current is balanced by the cathodic reduction of oxygen. Due to the size of the gap and slow diffusive motions within interstice, the oxygen is consumed and is not completely replaced from the outside. When all the oxygen is consumed within the interstitium, the metal is still passive, but the passivity current within the interstitium is balanced by the outside reduction of oxygen and thus there is a separation between anodic and cathodic areas. The passivity current continues to transfer metal ions through the passive film but, while outside the phenomenon is balanced by oxide reduction, within the interstice there is hydrolysis of metal ions and the migration of chloride ions from the outside, which generate acidity bringing the pH to values increasingly low. When the pH, because acid hydrolysis of the chloride ions, reaches a critical threshold, which depends on the intrinsic characteristics of the material, there is the breaking of the passive film and begins the phase of corrosion in depth with a speed controlled by the ohmic drops between anodic area (internal to interstitium) and cathode area (outside the interstitium).
- The corrosion fatigue manifests itself on the entire surface of the element subjected to cyclic stress and environmental aggression, with the appearance of cracks. The environments that favour the occurrence of this type of corrosion are the seawater and the solutions of chlorides. To prevent the phenomenon, it’s necessary to operate simultaneously in different directions: in the choice of the type of steel more suitable to use in the design phase and trying to minimize the vibratory phenomena that generate the states of cyclic stresses.
- The inter granular corrosion is a type of corrosion caused by aggressive agents which attack the grain boundaries of stainless steels, when, after the occurrence of thermal events, they prove to be sensitized. A stainless steel is sensitized when it remains for a short time also, at certain temperatures, which cause the loss of mechanical strength and toughness. The temperature considered harmful is between 450° C and 850° C for stainless steels austenitic steels, higher than 950° C for ferritic steels and between 250° C and 1300° C for austenitic steels stabilized.
7. Conclusions
Stainless steels are materials that are used for many applications in various fields in which it is required to resist the aggression of various environments. The knowledge of the main factors that determine the corrosion resistance and other aspects that can determine the trigger is essential for a correct choice of the right league. By contrast, recognize the type of corrosive phenomenon that occurred is surely basic to be able to properly select the type of steel to be used.
In view of the arguments outlined above, Cortem Group decided, for all equipment and products that need to be protected from such aggressive agents, to adopt the use of stainless steel of the austenitic type, of series AISI 304 and/or AISI 316L. This is in function of the applicability to the specific product and, in addition to comply with the required specifications of our customers, even after the verification of compliance with the chemical/physical to such requests, operating with a series of experimental analysis at our laboratory for internal testing or through laboratories external accredited.