Why an explosion accours
THE COMBUSTION It’s not so easy to cause an explosion or fire, at least theoretically. Combustion is the rapid transformation of chemical...
THE COMBUSTION
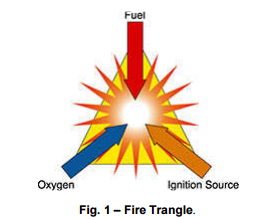
It’s not so easy to cause an explosion or fire, at least theoretically. Combustion is the rapid transformation of chemical energy into thermal energy. Oxidation, combustion and explosion are chemically exothermic reactions and only differ in reaction speed. In order to cause a reaction, three fundamental components have to be present at the same time:
- the combustible material - in the form of gas, vapours or dusts;
- the combustion agent - oxygen in the air;
- ignition energy - either electrical or thermal.
These three components form what is called the Fire Triangle (fig. n. 1). Once the reaction has been triggered, the result can be slow combustion, a rapid flame or an explosion, depending on how the exothermic energy is released. An explosion can be triggered due to plant causes (electric and not electric) only when combustible material, combustion agent and ignition energy are present in the environment at the same time.
MINIMUM IGNITION ENERGY
However, the presence of these three components of the fire triangle is still not enough to cause fire or an explosion. Firstly, the mixture consisting of the combustible material and the combustion agent must have a combination ratio within very specific limits. This ratio is the quantity of combustion agent, expressed in mass or volume, combined with the mass or volume of combustible material. Secondly, the ignition energy, measured in Joules, must exceed a threshold which is different for each substance. In practice, the ignition energy is a spark caused by some electrical phenomenon, such as those that occur, for example, opening the switch contacts. For any flammable substance is possible to draw a curve, as shown in Figure 2.2, which indicates the characteristic of ignition, that determines the minimum ignition energy, called M.I.E. (Minimum Ignition Energy), below which it is impossible to trigger the mixture.
EXPLOSIVE LIMITS
Looking at the figure 2, we can note that there are two limits in mixture concentration beyond which an explosion cannot occur:
1. as the concentration of combustible material in the mixture decreases, the energy required for ignition gradually increases to the point where ignition cannot occur due to the lack of combustible material. This point is called the Lower Explosive Limit (L.E.L.) and represents the concentration of combustible material in the air below which the atmosphere is not explosive.

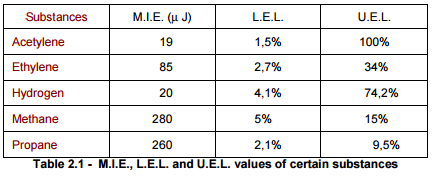
For example, in the following table (Table 2.1) we report the the M.I.E., L.E.L. and U.E.L. of certain substances. Other two important features of the flammable substances in order to determine their degree of risk and to classify them are the Flash point and the ignition temperature of which we discussed extensively in the newsletter of March 2011
IGNITION SOURCES
The most common source of ignition is represented by arcs and sparks caused by opening and closing contacts, for example, switches, remote control switch etc.. Sparks can sometimes be caused by loose terminals or by static electricity on plastic parts, such as equipment enclosures. The energy needed to ignite an explosive mixture is very low. In order to trigger a mixture of air and hydrogen are sufficient 20 microjouls, which are the energy of a spark produced by a current of 20 mA with a voltage of 10 V for the duration of 0.1 milliseconds. As we know, most of the electrical equipment exceeds these values during a normal use. The aim is to avoid the possibility of producing arcs or sparks that could ignite combustion, or where this is not possible, to ensure that they do not come in contact with the explosive mixture.